Why Your H&E Slides Are Cloudy & How to Correct It
H&E slides become cloudy because of insufficient clearing after the staining process. In this post, we explain why the cloudiness occurs during or after your H&E staining protocol and how to adjust your procedure to correct it for good.
Whether you’re using a regressive staining technique, such as Harris H&E, or a progressive technique, such as Gill’s H&E, excessive stain, moisture, and alcohol is removed throughout the process.
The basic steps of performing an H&E stain are:
- Remove paraffin wax using a solvent (xylene)
- Remove solvent using alcohol rinses
- Re-hydrate the specimen
- Apply hematoxylin to stain nuclei
- “Blue” the specimen further
- If using a Harris H&E: Differentiate using a weak alcohol
- Apply eosin as a counterstain
- Dehydrate in alcohol rinses
- Rinse in a solvent (xylene)
- Finish mounting the slide
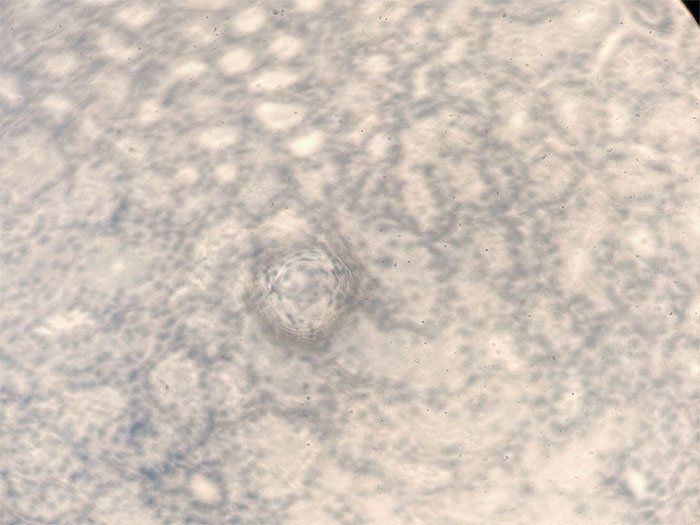
The final steps are to remove the excess water within the specimen using alcohol rinses, then expose the slide to a solvent to remove any remaining alcohol. This process prepares the slide properly for mounting. If the slide containing the specimen contains any water residue at this final stage, the slide will appear cloudy. The cloudiness will appear immediately and will present a blotchy cloudy appearance if coverslipped.
There are two main causes for cloudiness within an H&E-stained slide:
1. Insufficient stain clearing in the alcohol (insufficient dehydration)
Insufficient dehydration occurs after the eosin is applied as a counterstain. There are two possibilities for fixing this issue:
- Leave the specimen in the 100% alcohol rinse (step 8 above) longer. This will allow the alcohol rinse to remove all the excess stain and moisture, assuring an ideal specimen for mounting.
- Replace the alcohol rinses. If the 100% alcohol solution, in particular, hasn’t been replaced often enough, the solution can become diluted with water, which lowers the concentration of alcohol. If the percentage of alcohol is below the target concentrations, it will not remove excess moisture. Refresh the alcohol solutions to better dehydrate the specimen.
- Replace the xylene solvent. If any moisture has remained in the specimen as it was transferred to the xylene, or if the alcohol rinse had an improper ratio of alcohol to water in the final step before mounting, that water could be transferred to the solvent. This would also cause cloudiness in the slide. Refresh the solvent to properly clear the slide.
2. Inconsistent solution volumes in staining containers.
Cloudiness can also occur if the volume of liquids in the alcohol or xylene solution containers are not leveled to the proper depths. This could mean that the alcohol solution depths are not sufficient to cover the slide surface, and therefore, the alcohol doesn’t properly remove all moisture and excess stain that was existing on the surface of the slide. Fix this issue by returning the slide to the xylene solvent, then place it in fresh 100% alcohol solution at the appropriate depth. Finally, clear with fresh xylene and mount the slide.
Correcting your protocol to replace the alcohol solutions and xylene solvent solutions at regular intervals will help prevent this issue moving forward. This will also help your team maintain consistency when staining histology specimens.
If you have additional questions, or if you’re still experiencing issues with your slides after trying these corrections, our team is here to help! Call one of our microscopy experts at 856-224-0900 or email at [email protected].
Additional histology and cytology slide corrections are also available in our ebook, Lab Technician’s Guide to Troubleshooting Common Issues with Biological Stains. Download it today.