7 Tips to Stop False Gram-Negative Stains
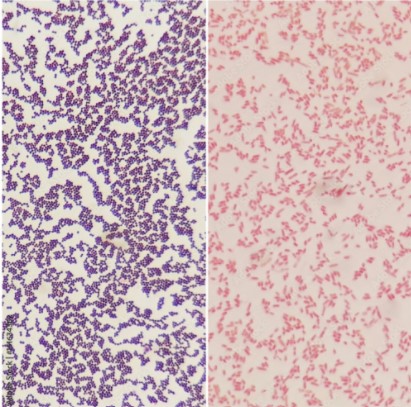
Gram staining allows us to differentiate bacterial species into two large groups, Gram-positive and Gram-negative, based on the properties of their cell walls. Gram-positive bacteria, such as staphylococci (“staph”), streptococci (“strep”), and pneumococci, retain the color of the crystal violet stain in the Gram stain and appear purple. Gram-negative bacteria, such as meningococci (bacterial meningitis) and the bacterial organisms that cause cholera, don’t retain the violet stain color in Gram’s method and instead take on the red color of the counterstain.
Typically, a misdiagnosis of Gram stains is due to issues with the stain protocol. These issues can cause Gram stains to decolorize too quickly (falsely suggesting Gram-negative bacteria) or too slowly (falsely suggesting Gram-positive bacteria). Try these tips to correct protocol-caused issues and stop your stain from decolorizing too quickly.
- Reduce heat during fixation – Gram staining works by first staining bacterial properties within the cell wall, then removing the excess stain using a decolorizer. Excessive heat during the fixation process alters cell morphology and breaks down cell walls. This can make cells more easily decolorized during the subsequent protocol steps. In order to reduce the risk of speedy decolorization, reduce the heat during fixation.
- Reduce water rinse time – Excessive washing can cause excessive decolorization. Crystal violet is susceptible to washout with water before binding to iodine, creating a false negative test. To avoid this issue, don’t use more than a five-second water rinse at any stage of the procedure.
- Increase iodine exposure – Insufficient iodine exposure due to lower concentration of iodine can affect decolorization. Keep iodine bottles closed when not in use. Open bottles lose more than 90% of available iodine in 30 days, while closed bottles lose about 50% in the same time frame.
- Reduce time in counterstain – By rinsing the slide with a counterstain, safranin, all cells are stained red. If the specimen is overexposed to the counterstain, however, it can replace the crystal violet-iodine complex in gram-positive cells. Reduce the time the specimen is exposed to the counterstain to under 30 seconds.
- Change your decolorization length of time – Reduce the time in the solvent and rinse quickly, or use a decolorizing solution that is easier to control for the decolorizing step. The acetone percentage determines the rate of decolorizing. 25% acetone is the slowest, and 75% acetone is the fastest.
- Read the QC slides – If the Quality Control slide is being over-decolorized, then most likely the specimen is also matching. Adjust your protocol accordingly.
- Examine acetone in your procedure – The main decolorizing factor in your protocol is the acetone. A 50/50 acetone decolorizer will decolorize much quicker than the 25/75 mix. If you switch suppliers or make your own decolorizer, the new decolorizer can impact performance. You will need to adjust the procedure to match your new decolorizing mix.
False Gram-negative stains can cause a plethora of issues for patients, so if these fixes aren’t working for you, please call the technical help team at 856-224-0900 or email at [email protected]
Read more on Gram stain protocol fixes and other common microbiology stain troubleshooting in our ebook, Lab Technician’s Guide to Troubleshooting Common Issues with Biological Stains.