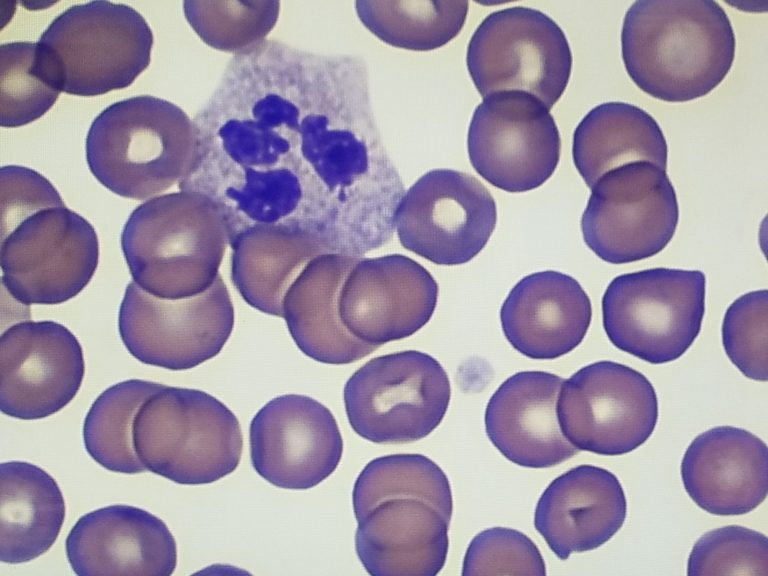
How To Improve Nuclear Staining in Histology Slides
Looking to improve nuclear staining in your histology slides? We can help! Histology specimens generally require a clear distinction between the nuclei and other cellular elements of tissue to give the pathologist the best appearance for diagnosis. Hematoxylin, a basic dye, stains the nuclei, giving it a blue-to-purple color. The eosin adds a pink tone to the cytoplasm and a reddish tone to the blood cells, resulting in contrasting blue-purple and shades of pink colors that are distinct within the cellular structures of the specimen.
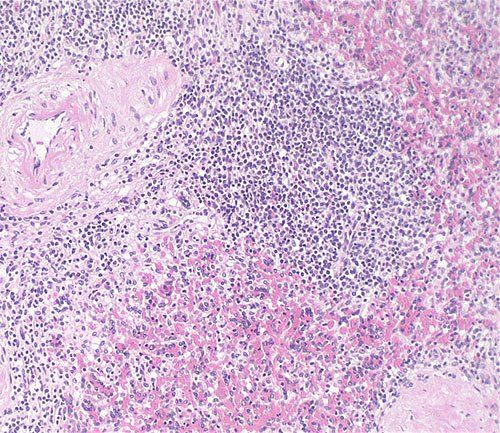
For routine diagnosis using histology specimens, the use of Hematoxylin and Eosin (H&E) stains is by far preferred for viewing cellular structure detail. Because this stain demonstrates such a broad range of cytoplasmic, nuclear, and extracellular matrix features, lab technicians are able to control the appearance and strength of basophilic staining to meet pathologists’ expectations. There are two main types: Harris H&E, a regressive technique, and Gill’s H&E, a progressive technique. This post discusses how to improve basophilic nuclear staining for each technique.
Harris H&E stain: basophilic staining is too weak
Harris hematoxylin is a regressive staining technique, so the tissue section is first overstained with hematoxylin to create blue cellular nuclei, then excess dye is removed during the differentiation step in a weak acid alcohol.
Weak basophilic nuclear staining in an H&E stain occurs when the hematoxylin’s color is too weak or the specimen wasn’t exposed to hematoxylin long enough. Here are the solutions we recommend:
- Exposure time in hematoxylin is too limited: To solve exposure time, adjust the length of time the slide is in the hematoxylin stain solution until you see the strength in staining you need.
- pH of hematoxylin is out of range: To solve for hematoxylin being diluted past the ideal range of pH 5-6, minimize any carry over of water to the hematoxylin solution during staining.
- pH of water out of range: Check pH of water prior to staining and use deionized or distilled water to replace tap water when possible. This can solve the issue of having too much chlorine in water which can act as a bleaching agent; weakening the hematoxylin stain.
- Weak solution penetration: For H&E stains on specimens that have been paraffin embedded, if the basophilic staining is too light, it is possible that the paraffin didn’t dissolve out of the tissue section completely. If there’s wax in the tissue section, the water-based staining solution can’t penetrate the specimen properly, leading to weak staining. Return slide to fresh xylene to remove paraffin wax from section then proceed with procedure to re-stain tissue specimen.
- Poor fixation or tissue processing: Another cause of weak basophilic staining is poor fixation or processing. This means that the stain doesn’t bind to the tissue; this is an issue with preparing the tissue.
- Acid alcohol concentration is too high: If the concentration of the acid alcohol is too strong for the differentiation step, or if the specimen spent too much time in acid alcohol, this regressive staining technique could result in too much of the stain being removed. Solve this issue by selecting an acid alcohol solution with a lower concentration (i.e. change from 1% to 0.5% solution) or decrease the specimen’s time in solution for this differentiating step.
Gill’s H&E Stain: basophilic staining is too weak
Gill’s Hematoxylin is a progressive stain. The stain is administered to the tissue section without the need of a subsequent step in a differentiator (generally, acid alcohol), to remove excess basophilic stain.
- Exposure time in hematoxylin is too limited: To solve exposure time, adjust the length of time the slide is in the hematoxylin stain until you see the intensity in staining you need.
- pH of hematoxylin out of range: To solve for hematoxylin being diluted past the ideal range of pH 5-6, minimize any carry over of water to the hematoxylin solution during staining.
- pH of water out of range: Check the pH of the water prior to staining and use deionized or distilled water to replace tap water when possible. This can solve the issue of having too much chlorine,which can act as a bleaching agent, in water, which can weaken the hematoxylin stain.
- Weak solution penetration: For H&E stains on specimens that have been paraffin embedded, if the basophilic staining is too light, it is possible that the paraffin didn’t dissolve out of the tissue section completely. If there’s wax in the tissue section, the water-based staining solution can’t penetrate the specimen properly, leading to weak staining. Return the slide to fresh xylene to remove paraffin wax from the section then proceed with the procedure to re-stain tissue specimen.
- Poor fixation or processing: Another cause of weak basophilic staining is poor fixation or processing. This means that the stain doesn’t bind to the tissue; this is an issue with preparing the tissue.
We produced an eBook, The Lab Technician’s Guide to Troubleshooting Common Issues with Biological Stains, to help lab technologists adjust their staining techniques to better see differentiation and varying strengths, as well as troubleshoot common issues that arise in histology, microbiology, cytology, and hematology specimen stains. Download now to learn more!