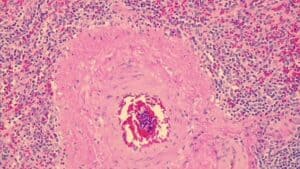
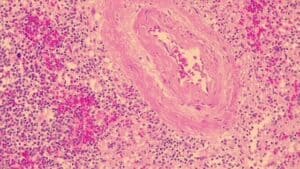
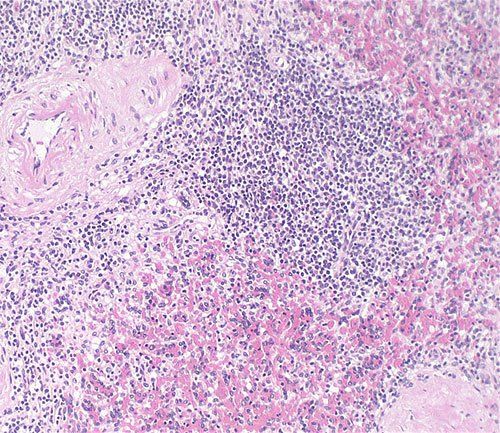
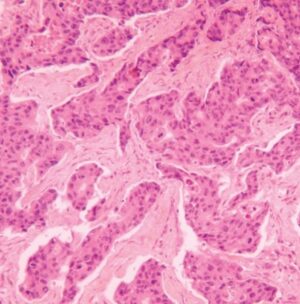
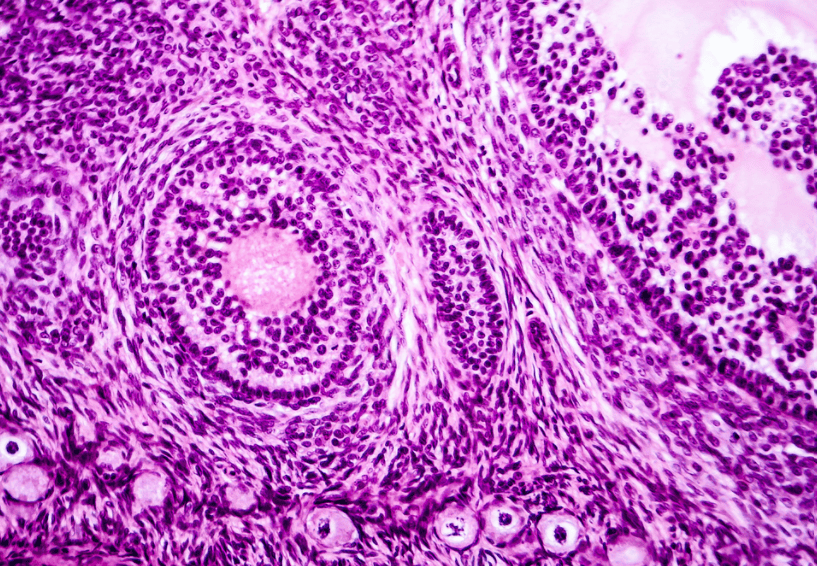
How To Set-Up And Conduct Vibrant H&E Stains
Used for many histology specimen stains, H&E stains appear darkest/most intense in appearance. Since the appearance of a slide is somewhat subjective, use this stain if the Gill 3X hematoxylin isn’t dark enough for your histology stains. Alternatively, use the Harris, Acidified progressive method (found below) for fewer steps and the same good quality.
Protocol for Harris H&E stain for histology
- Paraffin slides: Deparaffinize in xylene, then rehydrate through graded alcohols to water.
Frozen slides: Fix in 95% Ethanol (or other fixative according to lab preference) and rinse in water.
- Stain in Hematoxylin Solution, Harris: 4 to 6 minutes
- Rinse in two changes of DI water: 1 minute each
Note that this could also be done using running tap water, but we recommend DI water to avoid the varying pH tap water can have.
- Acid Alcohol 0.5%, or Acid Alcohol 1%: 10 seconds
Note some prefer to use a weaker acid alcohol rinse which is 0.5% while others prefer the 1% acid alcohol rinse. The higher percent solution will extract dye a little fast but there is not much difference from the weaker solution here.
- Rinse in two changes of DI water: 1 minute each
Note again that this could also be done using running tap water.
- AquaBluing Reagent (until tissue turns blue): 30 to 60 seconds
- Rinse in two changes of DI water: 1 minute each
Note again that this could also be done using running tap water.
- Ethanol 95%: 15 to 30 seconds
- Eosin Y 1% Alcoholic, or Eosin Y Intensified, or Eosin-Phloxine: 1 to 2 minutes
Note: for an increased definition of cytoplasmic components over Eosin Y 1%, select Eosin Y Intensified or Eosin-Phloxine as a substitute.
- Dehydrate in Ethanol 95%, two changes: 10 dips each
- Dehydrate in Ethanol 100%, two changes: 1 minute each
- Clear in Xylene or Xylene substitute, two changes: 1 minute each
- Coverslip using Acrylic Mounting Medium and examined under microscope
The Harris Hematoxylin, Acidified stain is a progressive staining technique that takes fewer steps and still has the more intense, darker purple color of the Harris H&E stain. It can be used when histology specimens aren’t intense enough for your pathologist’s preference, as well as in some instances for cytology specimens when the Gill 1X isn’t intense enough.
Protocol for Harris Hematoxylin, Acidified stain for histology
- Paraffin slides: Deparaffinize in xylene, then rehydrate through graded alcohol to water.
Frozen slides: Fix in Ethanol 95% (or other fixative according to lab preference) and rinse in water.
- Stain in Hematoxylin solution, Harris Hematoxylin, acidified 2 to 4 minutes
- Rinse in two changes of DI water: 1 minute each
Note that this could also be done using running tap water, but we recommend DI water to avoid the varying pH tap water can have.
- AQUABluing Reagent (until tissue turns blue): 15 to 60 seconds
- Rinse in two changes of DI water: 30-60 seconds
Note again that this could also be done using running tap water.
- Ethanol 95%: 15 to 30 seconds
- Eosin Y 1% Alcoholic, Eosin Y Intensified, or Eosin-Phloxine: 30 to 60 seconds
Note: for an increased definition of cytoplasmic components over Eosin Y 1%, select Eosin Y Intensified or Eosin-Phloxine as a substitute.
- Dehydrate in Ethanol 95%, two changes: 10 dips each
- Dehydrate in Ethanol 100%, two changes: 1 minute each
- Clear in Xylene or Xylene Substitute, two changes: 1 minute each
- Coverslip using Acrylic Mounting Medium and examine under microscope
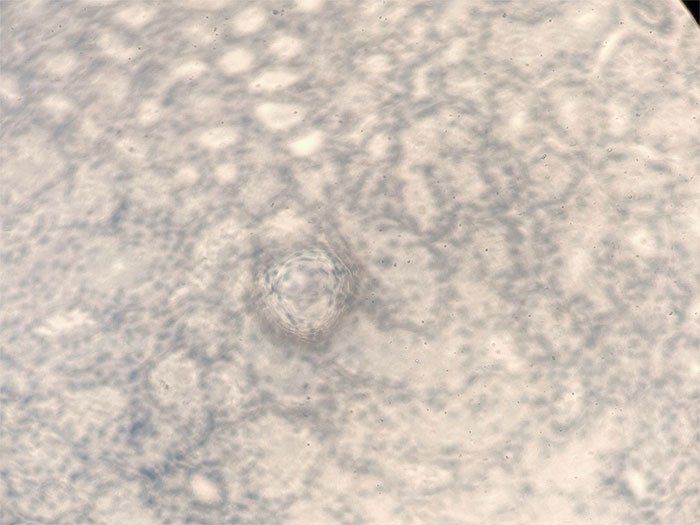
Occasionally, you will see cloudiness in an H&E slide once you’ve completed the protocol. There are two main causes for this:
- Insufficient dehydration
- Inconsistent solution volumes
Our experts explain how to solve this issue in our post, “Why Your H&E Slides Are Cloudy & How to Correct It.”
For more information on the two protocols in this post, or for other protocols in a quick reference format, download our ebook, Quick Reference Guide to Common Laboratory Biological Stains.