How to Set-Up and Conduct an Acid Fast Stain
Acid fast bacteria (AFB), bacteria high in mycolic acid (lipids in the cellular envelope), are detected using an Acid Fast Stain. These stains differentiate the AFBs, which cause diseases such as tuberculosis and other respiratory illnesses. AFBs are typically tested for in a special laboratory environment because these diseases are highly contagious. Whether you’re testing surgical specimens, respiratory or sputum specimens, the two counterstains in these protocols are either brilliant green or methylene blue; to provide different looks to the non-acid fast organisms. Your pathologist’s preference will dictate which color you should use with the Acid Fast Stain.
Additionally, there are two variations to this stain – the cold method ( Kinyoun) or the hot method (Ziehl Neelsen). The original Ziehl Neelsen method is more traditional, while the Kinyoun method is growing in popularity due to not requiring heat to work.
Acid Fast stain
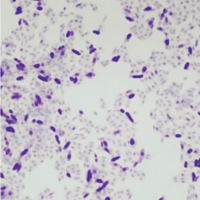
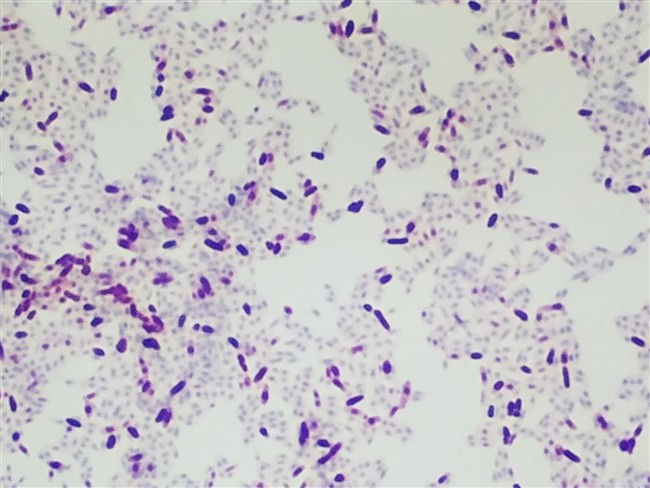
Acid Fast stains are used for identifying acid fast organisms. These bacteria will be stained red by the protocol.
Protocol for Acid Fast (Kinyoun or Ziehl Neelsen) stain
- Fix the slide.
- Kinyoun (cold method): Flood slide or submerge in the Kinyoun Carbol Fuchsin solution: 5 minutes Ziehl Neelsen (hot method): Flood slide or submerge in Ziehl Neelsen Carbol Fuchsin solution and heat: 3 minutes
Can use a slide holder or stain individual slides.
- Rinse gently with DI water.
- Remove most of the stain using the Decolorizer solution until the washings are clear.
- Rinse gently with DI water.
- Immerse the slide in the Methylene Blue solution or Brilliant Green solution for 30 seconds to 1 minute.
- Rinse gently with DI water and allow to air dry.
- Examine the slide accordingly.
Acid-fast organisms are stained red, while non-acid-fast organisms and the background are stained green or blue. A major characteristic of mycobacteria is that they are “acid-fast” and once stained with aniline dye, basic fuchsin, they are difficult to decolorize, thus retaining the red color even when exposed to an acid alcohol rinse.
Carbol fuchsin is used as the primary dye for acid-fast organisms because it is more soluble in the cell wall lipids than in the acid alcohol, so the organism will retain the dye’s red color. If you’re seeing an issue with your acid fast organisms not retaining color as expected, read our post on how to fix an acid fast stain that’s not retaining dye.
The Kinyoun method can also be modified as a weak acid fast stain, which uses 5% sulfuric acid instead of hydrochloric acid. The weak acid fast stain, in addition to staining mycobacteria, will stain organisms that are not able to maintain the carbol fuchsin after decolorizing.
Learn more about Acid Fast Stains and other microbial stain protocols in our Quick Reference Guide to Common Laboratory Biological Stains. Download it today!