Choosing the Right Fixative
When selecting a fixative for your specimen, whether it be histological or cytological, preserving tissue structure to prevent degradation of the cells and tissue is imperative.
It’s important to select the proper fixative for the tissue, but it’s equally important to ensure the ratio between specimen and fixative is adequate to do the job. The recommended ratio is 1:20 specimen to fixative. A greater volume of fixative to specimen is fine, however, a lower volume of fixative may leave the specimen poorly fixed. If fixation is inadequate, the subsequent steps will not be able to compensate for this. It is essential to take the time to select the proper fixative for your specimen to assure quality end results.
To determine the right fixative to use, there are a few questions to consider
- What is your specimen type?
- What components do you want to demonstrate?
- What type of staining procedure do you intend to use on the specimen to obtain results?
First, let’s break down the various specimens and the fixative options you have available to choose from in each instance.
Soft Tissues
Soft tissue, such as heart, lung, liver, kidney, and GI specimens, can use a universal fixative, 10% neutral buffered formalin (NBF). In particular, this fixative can be used for most specimens requiring paraffin embedding.
Although 10% NBF is considered a universal fixative and provides exceptional results, it may not be appropriate for all specimen types.
The 10% neutral buffered formalin fixative provides exceptional preservation of tissue and contrast between different tissue elements when observing it after H&E staining. If your specimen will be undergoing staining using Gill or Harris H&E staining, this is often the correct choice. Learn more about these procedures in our ebook, The Quick Reference Guide to Common Laboratory Biological Stains.
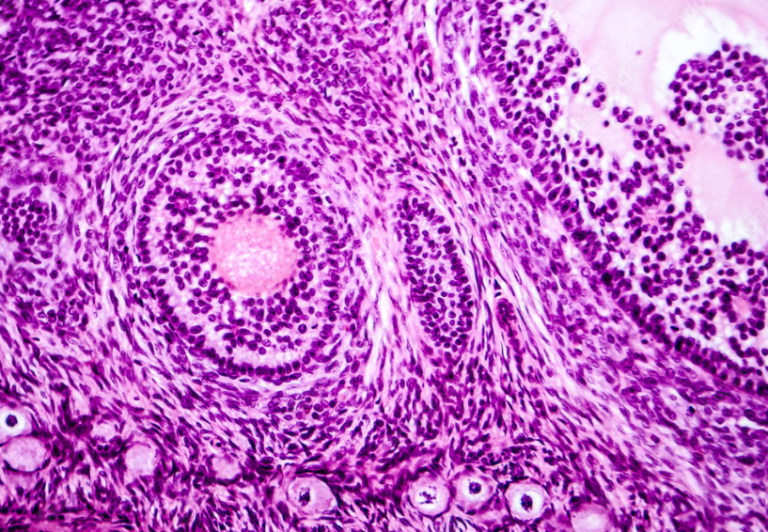
Hard Tissues
Hard tissue, including bone, sternum, cartilage or bone marrow trephine, can use either the universal fixative, 10% NBF, or 10% formalin (unbuffered).
It should be noted that unbuffered formalin, however, slowly oxidizes into formic acid. As it oxidizes, the pH begins to fall, which causes a reaction between the formic acid and hemoglobin to produce unwanted brownish-black pigment on blood-rich tissue, such as bone marrow. This pigment can cause a challenge to read through and may even cause misdiagnosis of tissue specimens or confusion with microorganisms. Although this pigment can be removed from tissue specimens with saturated aqueous picric, some people prefer to go with the buffered 10% formalin to avoid this possibility.
Since these fixed specimens will be processed for paraffin embedding, it will likely undergo decalcification next. After suitable fixation and decalcification, the specimen is ready for processing and embedding. These steps will most likely result in a routine stain) using Gill or Harris H&E protocols.
Fatty Tissues
Fatty specimens, such as breast tissue, can be sufficiently fixed in 10% NBF. An efficient alternative to 10% NBF is using a fixative that is a blend of buffered alcohols to stabilize the cellular components and provide a life-like appearance. We offer a solution called TRIPLEfix which is formulated specifically for fatty specimens.
Follow the protocol for histological stains using either progressive or regressive stains. For a more intense nucleic acid staining, choose from the progressive stains of Gill 2X or 3X hematoxylin. For a more intense purple color, you can also select Harris Hematoxylin. By the simple addition of acid to the stain formulation, the Harris becomes a progressive stain and can also be used to provide intense staining, when selecting Harris Hematoxylin, Acidified.
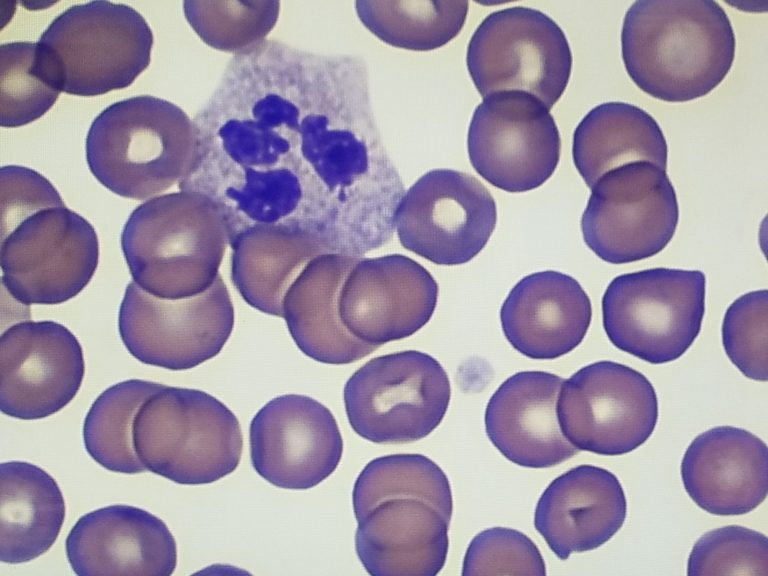
Lymphoid and Hematopoietic Tissues
For these specimens, a mercury-free fixative, such as B-5 Substitute, is appropriate to use. This substitute for mercuric chloride produces excellent preservation of cellular detail and provides good results with many stains, including special stains.
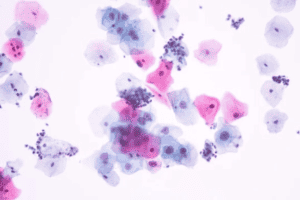
Cytological Specimen
Alcohols, specifically ethanol, are used primarily for cytologic smears. For exceptional quality, we recommend you complement the alcohol with a ready-to-use cytology fixative such as our Saccomanno Fluid. Ideal for specimens such as fine needle aspirates (FNA’S), bronchial washings, pleural and peritoneal fluids, as well as urine and sputum, this fixative is recommended as a pre-fixative, followed by 95% ethanol solution fixation. Learn more about this protocol in our ebook, The Quick Reference Guide to Common Laboratory Biological Stains.
These fixative options are all referenced in our ebook, The Quick Reference Guide to Common Laboratory Biological Stains. This guide follows a decision-tree, enabling you to make the best choice on stains for your laboratory. Download it today!