LATERAL FLOW ASSAY DEVELOPMENT & MANUFACTURING
For more than 20 years, the American Bionostica (ABI) team has manufactured protein based lateral flow diagnostics for OEM clients in food safety, agricultural, environmental, veterinary medical, biodefense and clinical sectors. Immunoassays are manufactured using colloidal gold and polymer (“latex”) fluorescent nanoparticle conjugates to support visual and quantitative LFD reader technologies. With its acquisition in 2021, ABI/Ethos Biosciences capabilities have expanded to include europium chelate microsphere formats for use with time- resolved fluorescence readers.
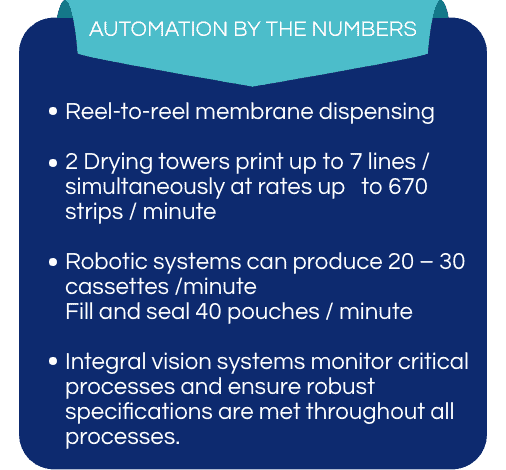
Our highly-specialized lateral flow assay development and manufacturing operations are located within the 126,000 square foot Ethos Biosciences facility in NJ, and include reel-to-reel dispensing and drying, sensor-based test strip cutting, reel-to-reel lamination and robotic cassette assembly and pouch filling/sealing equipment. Critical processes are performed in a customized Dry Room with state-of-the art dehumidification system. Our QC laboratory is designed to support achievement of your specifications.
OFFERING:
Fluorescent Nanoparticles | Dyed Nanoparticles | Colloidal Gold | Membranes | Pads | Cassettes | Buffers
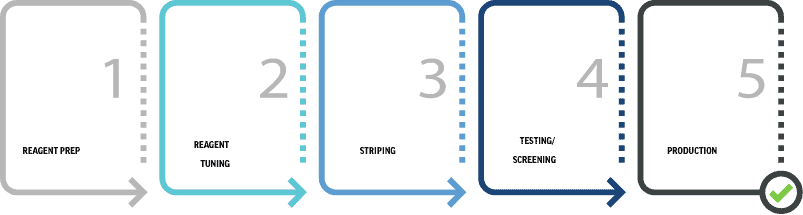
LATERAL FLOW CMO PROCESS
Our lateral flow CMO process is designed for seamless project onboarding and optimal manufacturing outcomes.
We work in partnership with Bangs Laboratories, a leading manufacturer of polymer, silica and magnetic microspheres with 35 years of experience supporting
IVD clients through microsphere synthesis and fine particle analysis.
Ask about our nanoparticle reagents and lateral flow device consumables for initial development and proof-of-concept work.
WE OFFER:
- An experienced team to transfer your technology to our manufacturing and QC processes
- Manufacturing troubleshooting, optimization & validation
- Continual project reporting and client collaboration
- Prequalification testing and assay samples prior to locking manufacturing processes and specifications
- Scale-up capability

